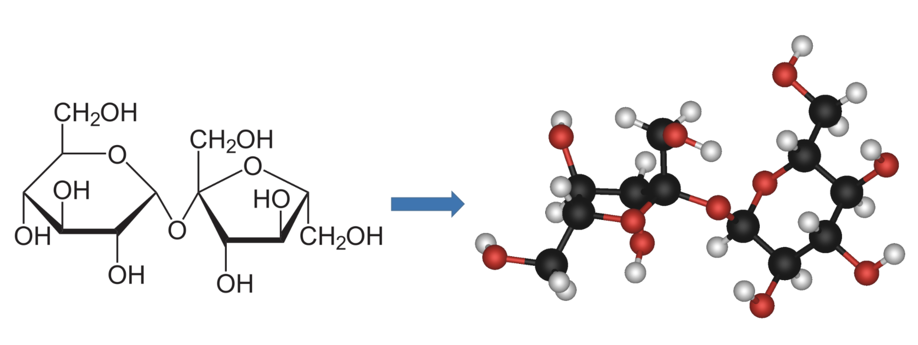
Table 1: The illustration of sucrose (table sugar) changes from a 2-D image on paper (left) to a 3-D object (right) that can be rotated and visualized from a variety of angles by means of computer modeling. Source: Author’s own.
Why does water boil at 100oC and alcohol at just 78oC? Why is graphite soft and diamond hard if they are both made of the same element, carbon? These and many other questions related to the behavior of substances can be answered by examining the structure of the building blocks of matter: atoms and molecules.
The structure and properties of matter depend on the way in which electrons are distributed around atomic nuclei. Although general chemistry covers this knowledge, the physical-mathematical details that occur in this relationship go beyond the scope of pure chemistry and are linked to physics, in particular quantum mechanics, which is a branch of science that describes the behavior of matter at the microscopic level.
data-animation-override>
“Molecular modeling remains unexplored in basic chemistry courses, perhaps because instructors have not yet realized the benefits of tools like GAMESS, Gaussian or Q-Chem and its usefulness in education.”
Even though the basic equations of quantum mechanics used to describe molecules can be presented in a simple way, solving them is a complicated matter. In fact, it is so complex that to date only exact solutions have been found for a very small number of molecules, all of them with just one electron.
This is clearly not enough: water has 10 electrons, whereas a molecule of sucrose (table sugar) has 244. Consequently, chemists and physicists have resorted to finding approximate solutions for these equations, using their results in the prediction or explanation of experimental observations.
Reaching an approximate solution in a molecule requires the use of specialized programs such as GAMESS, Gaussian or Q-Chem, which, thanks to technological advances, are now available to university students, who can model molecules by installing these programs on their laptops. Twenty years ago, this would have been impossible since the computer capacity needed only existed in the most powerful computers of that time.
Despite these technological advances, molecular modeling remains unexplored in basic chemistry courses, perhaps because instructors have not yet realized the benefits of this tool and its usefulness in education.
data-animation-override>
“By modeling carbon dioxide we can intuit why this molecule contributes to global warming; by modeling the denaturation of albumin we can discover why an egg “gelatinizes” when placed in a hot frying pan.”
Gaussian, which has a highly user-friendly interface and is widely used among specialists in the field, can be installed on any laptop. The program finds approximate solutions for molecules of all sizes and, therefore, facilitates the learning process. For example, by modeling carbon dioxide we can intuit why this molecule contributes to global warming; by modeling the denaturation of albumin we can discover why an egg “gelatinizes” when placed in a hot frying pan; by modeling a chemical reaction we can understand why certain products are formed and not others, and so on.
Gaussian is the fruit of the work of Walter Kohn and John Pople, who developed the Density-Functional Theory (DFT) and other computational methods that earned them the Nobel Prize in Chemistry (awarded in 1998). DFT is one of the great ideas that triggered the breakthrough in approximate quantum mechanics solutions for large molecules, such as polymers (resins and plastics) and biopolymers (proteins and carbohydrates).
To demonstrate its usefulness, Gaussian was applied at the recent National Chemistry Education Congress for the real-time modeling of the infrared spectrum of acetic acid with DFT, as well as the nuclear magnetic resonance spectrum of nitroaniline.
Teachers who would like to use these types of programs in the classroom should bear two things in mind: 1) a clear purpose of use, and 2) a suitable methodology for the complexity of the intended modeling.
Regarding the first point, the teacher must be clear from the start why the molecule is being modeled: For students to visualize it? To optimize their geometry? To understand the types of links that are present? To understand a chemical reaction? The purpose of use determines the method to be employed.
As for the second point, the time it takes to make a computational calculation can vary from a few seconds to several hours, depending on the size of the molecule and the extent of accuracy sought in the solution. Therefore, it is important for the teacher to develop an idea, however vague, of the accuracy they wish to achieve within a reasonable calculation time, according to the vision and scope of the course in which the program is being used.
Finally, the use of programs should not act as a distraction from the learning objectives of the course. Given its ease of implementation, we must avoid using modeling as a “black box” that prevents us from reflecting on what the program is doing and properly interpreting the results obtained.
The idea that electrons flowing through the circuits of a computer can be used today to understand the behavior of electrons in the atoms and molecules of every type of material imaginable is intriguing: electrons modeling electrons.
Teachers who would like to learn more about this program and explore its capacities for basic chemistry education, please do not hesitate to contact me.
About the author
Alejandro Parra Córdova earned a Ph.D. in Chemistry from Binghamton University. He specializes in polymer physics and chemistry He is also a professor at Tecnológico de Monterrey, Campus Guadalajara.
This article from Observatory of the Institute for the Future of Education may be shared under the terms of the license CC BY-NC-SA 4.0 
)
)
)